High-purity water systems often go unnoticed — until they fail.
When they do, they disrupt analyser uptime, delay results, and increase stress for lab staff.
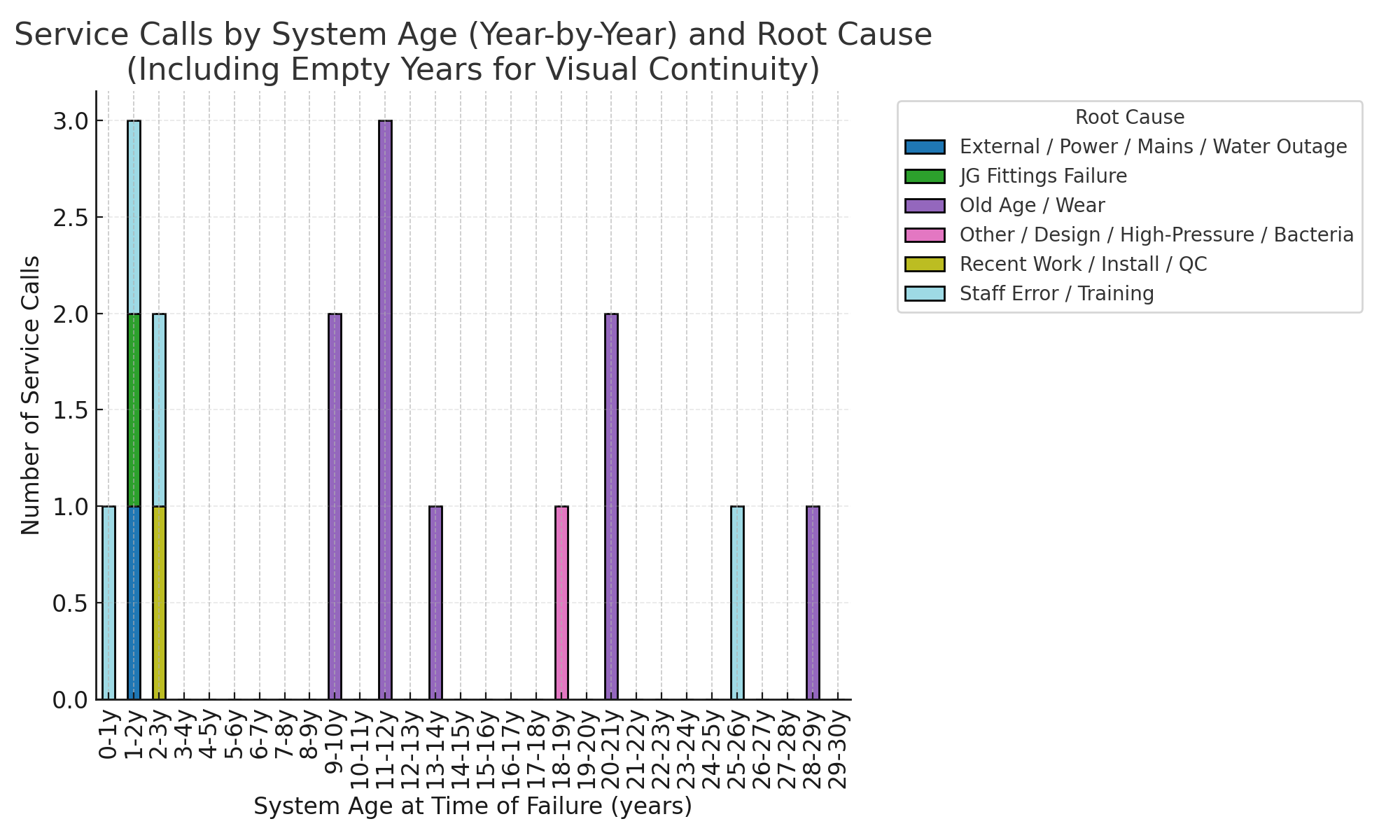
To understand how reliability changes over time, we analysed our call logs calls and mapped them against the age of the machines.
The year-by-year chart shows a clear pattern.
What the Data Shows
Early Years (0–3)
Most calls were not hardware failures but:
- Staff mistakes during filter changes or leaving the RO unit off.
- Minor install or QC oversights.
- External factors such as power outages or low mains pressure in the building.
These can largely be prevented with better install checklists and simple staff guidance.
Middle Years (4–7)
Units in this range are generally stable and low-cost provided they receive routine preventive maintenance.
Wear-Out Years (7–10)
Calls begin to rise as components age:
- Pump heads and seals.
- Probe assemblies.
- Float switches and O-rings.
Proactive part swaps from year 7 can prevent many emergency visits.
Reliability Spike (10–12)
This is the peak for service calls, dominated by age-related failures of pumps, probes, fittings and filter heads.
For high-throughput labs, planned refurbishment or replacement is often more cost-effective than repeated urgent repairs.
Long-Life Tail (15 + Years)
Legacy units still running often need multiple interventions per year and carry higher downtime risks.
A retirement or rebuild plan for units over 15 years old is essential.
Root-Cause Patterns
- Wear-out failures dominate after year 7.
- Staff errors and install issues cluster early in life.
- External mains and power issues appear at random across all ages.
Practical Actions
- Start enhanced checks and part swaps at year 7.
- Keep common wear items in stock to cut downtime.
- Provide a simple quick-reference card or short videos to reduce early-life errors.
- Use age-band data to plan refurbishment or replacement cycles.
The Take-Home Message
Early-life issues are mostly preventable with good installation and training.
True wear-out starts around year 7 and peaks at 10–12 years.
By acting early and planning ahead, labs can avoid most urgent failures, lower lifetime costs, and keep analysers running when they’re needed most.